Application Story: Saving lives with low-cost molecular diagnostic pathogen tests
By Festo Canada
Features DHOE head diagnostics EXCM planar surface Fast MDx festo gantry pathogens
The Fast MDx testing system is not only a game-changer in pathogen detection, it promises to be a life saver. Available starting in June, Fast MDx is the world’s first fully automated, high-throughput, near-patient molecular diagnostic testing system.
Developed by the London, UK-based social enterprise company of the same name with engineering support and automation technology from Festo, this extremely compact, mobile platform cuts the typical 24-48-hour turnaround time for test results to just 1-2 hours.
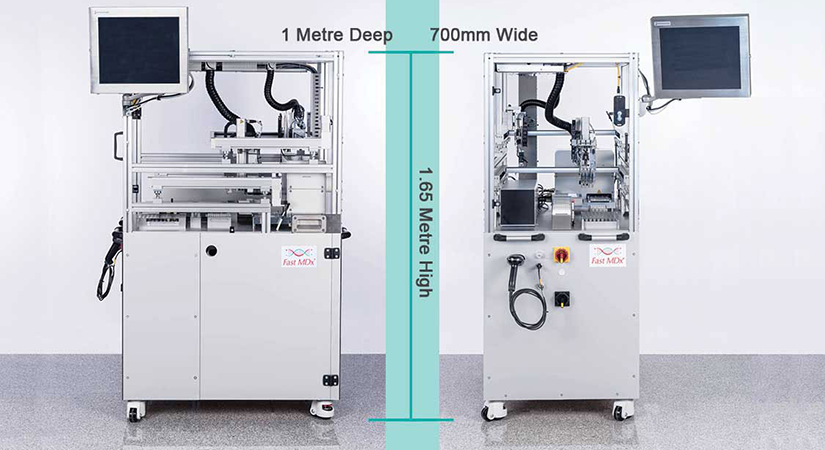
Above left: In the new near-patient Fast MDx molecular diagnostic testing system, Festo automated pipetting apparatus — a DHOE pipetting head and DHAO disposable syringe tip ejector — pierces the Pathtube cap to aspirate and pipette each patient sample. Above right: The compact, mobile Fast MDx is the world’s first fully automated, high-throughput, near-patient molecular diagnostic testing system, developed with Festo engineering support and technology.
Faster results mean earlier treatment and the possibility to contain infectious disease spread.
Watch video demonstration:
Fast MDx requires just a single technician, who can process 1,000-plus samples during an eight-hour shift. The company says that’s five times more efficient than manual test processing. Fast MDx platforms will be set up close to patients in hospitals or clinics or in large medical practices.
Not having to ship test samples to a central biosafe lab saves time and money on transport and use and disposal of specialized packaging. “Infected people receive faster, personalized treatment,” says Richard Lewis, founder and CEO of Fast MDx.
“Thanks to the close cooperation between Fast MDx and Festo, we were able to build the first prototypes quickly and efficiently, despite the extremely high degree of complexity,” says Lewis. “We were able to combine Fast MDx’s extensive expertise in qPCR thermal cycling with Festo’s proven 3D gantries, controllers and pipetting systems.”
Initially, Fast MDx will offer approved tests for common respiratory pathogens: influenza A/B, RSV A/B and Covid-19. Over the next 1-2 years, additional tests are planned for healthcare-acquired infections such as C-Diff and MRSA, as well as for sepsis, which kills millions worldwide every year. Eventually, genomic tests might be added that can detect hereditary diseases at an early stage.
As a social enterprise company, Fast MDx will lend out the platforms for free. The reagents and other supplies will be the principal expense, offering a low-cost alternative to centralized test processing, says Lewis. “That makes it the first system to make near-patient, molecular testing and diagnostics accessible everywhere, not just in more affluent countries.”
Fast MDx is a fully integrated system that covers every stage of test processing, including the electronic transmission of results. It has a very small footprint – 0.7m x 1m – and sits on lockable casters, so it can be relocated as needed.
Each unit includes two Festo EXCM planar surface gantries with electric axes for precise pipette handling and for robot-assisted handling of the PCR microtiter plates. The operator inserts three racks of pipette tips and a 96-well microtiter plate into the Fast MDx platform.
The assay kit with all reagents and controls is loaded into an aluminum block and clipped into place. 92 patient samples that have been collected in Fast MDx’s patented Pathtubes are loaded and tracked throughout the process using a unique barcode, laser-etched on the bottom of each Pathtube.
During sample preparation, an EXCM gantry operates in the X and Y planes. First, the Pathtubes are scanned using Festo optical sensors. The automated pipetting apparatus – a Festo DHOE pipetting head and DHAO disposable syringe tip ejector – is mounted on the gantry in Z direction. The disposable tip pierces the Pathtube cap, and aspirates and pipettes the sample. Each tip is used for one sample, then discarded.
“Piercing the Pathtube cap saves a lot of time and money, as no conventional opening and closing systems are needed, which eliminates human handling errors that so often happen with manually pipetting hundreds of samples,” says Hannes Rößer, handling technology expert at Festo.
In the next steps, an EXCM-30 gantry with an EHPS electric gripper picks up the filled microtiter plate and places it in a heat sealer for sealing with a plastic film. Then it is on to the RT block, which triggers the conversion of the RNA, if present, into complementary DNA (cDNA). Ultrafast fluorescent detection determines whether the cDNA sequence of the pathogen of interest is present in a sample.
With Festo’s PGVA pressure and vacuum generator on board, Fast MDx doesn’t require an external compressed air supply for pressure-over-liquid pipetting. PGVA only needs 24-volt power to produce pressure or vacuum of ±0.5 bar.
Dubbed the “Magic Box” by another Festo life sciences customer, PGVA integrates compressor, air preparation including filtering, reservoir and electronic pressure and vacuum control in a package measuring only 210 x 76 x 208 mm.
The 50-decibel pump generates pressure or vacuum with a fast, energy-efficient piezoelectric regulator that controls the flow of air, filtered to 0.001 µm purity.
Print this page