
Fittings and valves avoid corrosion with tantalum chemical vapour deposition
Mike Edwards
Features corrosion fittings valves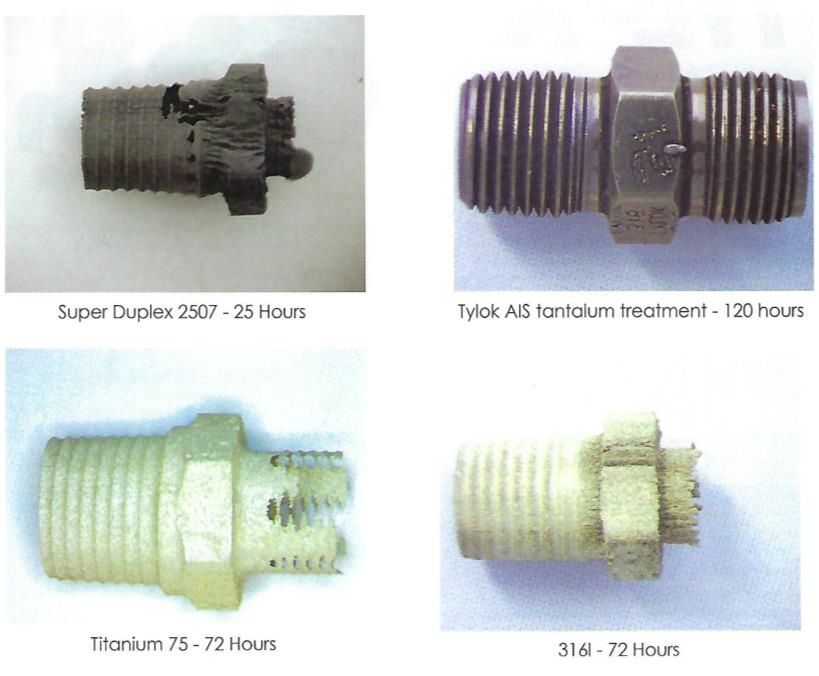 Components tested in aqueous 25% hydrochloric acid at 75°C for various time durations.
Components tested in aqueous 25% hydrochloric acid at 75°C for various time durations. The chemical vapour deposition (CVD) of tantalum over stainless steel fittings and valves is an innovative solution for both the toughest applications for corrosion resistant alloys (CRAs) and the problems of interchangeability between exotic alloys.
By Jesse Henning, Alanna Reis-Murray, and Natasha Reis-Murray
The drivers of North American manufacturing and industrial processing include chemical processing, oil and gas, pharmaceutical, semiconductor, mining, and marine industries. These industries often involve extreme environments and corrosive media, and therefore require specialty components above the standard copper, brass, steel, and stainless steel commonly used in tubing, fittings, valves, etc.
Various exotic alloys have been introduced to cope with these highly corrosive environments, including Hastelloy, titanium and zirconium. They are often made from precious or rare metals, often making exotic alloy parts expensive and harder to source than traditional materials. Additionally, sometimes a need exists for different alloys within the same system. As the industry grows, so does the need for innovative solutions in these complex systems.
Tantalum – A New Possibility
The element tantalum is a highly expensive exotic metal that has many of the properties desired in these extreme and aggressive environments. It has a melting point of 3017°C/5463 F, is biologically inert, and offers improved sealing in corrosive environments. It is the most corrosion resistant metal commercially available, and is particularly suitable in acidic, corrosive, and oxidative environments. Tantalum has been tested for corrosion resistance and has been proven to be extremely corrosion resistant in the majority of common industrial media requiring the use of exotic alloys. With the exception of fluoride and fuming sulfuric acid, it performs well in all acids. For example, in hot acid chloride environments tantalum outperforms c-276, Alloy 22, titanium and zirconium.
The American Society for Metals (ASM) Handbook on Tantalum:
“Tantalum is one of the most versatile corrosion-resistant metals known. It combines both the inertness of glass, the strength and ductility of low-carbon steel, and a much higher heat-transfer capability than glass. The relatively high cost of tantalum has been a limiting factor in its use, but new fabrication techniques, in which thin linings of tantalum are used in chemical vapor deposition processing equipment, resulting in components that have all the acid corrosion resistance provided by tantalum but at a much lower cost than an all-tantalum construction.” (ASM Metals Handbook, Vol 13B, 2005)
Tantalum Infused Chemical Vapour Deposition Process
Pure tantalum fittings and valves would be cost-prohibitive, so to exploit its desirable properties often layers of tantalum are coated over standard items. However, an improved method of combining tantalum with traditional metals has been developed. Tylok International, an Ohio-based manufacturer of stainless steel compression fittings and valves, has partnered with Tantaline, a CVD materials corporation, to create Tylok Alloy Interchangeability Solutions (Tylok AIS), a cost-effective and short lead timeline of tantalum diffused stainless steel parts.
Using a proprietary Chemical Vapour Deposition (CVD) technique, standard stainless steel fittings and valves are treated with Tantalum to alloy-bond the metals. This creates a thin 50 µm layer of 99.9% pure tantalum on 316/316L stainless steel. This technique offers many benefits over traditional coating and cladding techniques.
A Comparison between CVD and Traditional Techniques
This Tantalum diffused CVD technique is superior to other methods, i.e., coating or cladding, because the tantalum is embedded in the substrate, usually 316/316L stainless steel, forming an alloy zone between the substrate and outer tantalum layer. This is in contrast to traditional coatings, which may suffer from adhesion, spalling or flaking issues due to mechanical or chemical stresses.
With CVD, the inert tantalum surface does not suffer from these same failure mechanisms because it is fully embedded rather than coated and provides an impervious and inseparable barrier that completely isolates the substrate from the corrosive system fluids. This allows the cost effectiveness and physical strengths of 316/316L stainless steel to be combined with the corrosion resistance of tantalum. CVD is also geometry-independent so the internal and external faces of complex shapes may be treated, as this is a gas-phase technique. This impenetrable layer is only 50 µm thick, an advantage over the thick sheets required for secure cladding.

Galvanic series of metals. Corrosion resistant alloys used in fluid systems are shown in the boxed region.
Alloy Interchangeability
One of the more exciting applications of this technology is its use as an interchangeable replacement part in existing systems using exotic alloyed fittings and valves. Tantalum treated parts are marketed as interchangeable with other exotic alloys, which reduces the difficulties in sourcing replacement parts in existing systems.
The industry standard is to avoid intermixing alloys to reduce the possibility of galvanic corrosion, but this is not always feasible or desired because of the complexities and cost of sourcing all components in a single exotic alloy. Careful planning and design considerations can allow the use of dissimilar metals within a single system where galvanic corrosion will not occur.
Galvanic Corrosion – A Key Concern
Galvanic corrosion occurs when an electrolyte solution causes ion migration between two dissimilar metals in contact with each other, causing one metal to corrode faster than it would otherwise. Ions flow from the more active (less noble) metal to the less active (more noble) metal causing the more active metal to corrode faster than it would alone while the less active one corrodes slower.
The less noble metal is referred to as the anode and the more noble metal is referred to as the cathode. Whether corrosion occurs and which metal corrodes depend on the relative electrode potentials of the metals, as outlined in the Galvanic Series. As observed, commonly used exotic alloys are clustered on the noble portion of the table and are cathodes.
Galvanic Corrosion – The Solution
Corrosion is primarily dependent on the difference in electrode potentials of the metals, and the relative surface areas of the anode and cathode. By taking all these factors into consideration, dissimilar metals and alloys can be used in a system without fear of galvanic corrosion.
First, there must be a sufficient difference in electrode potential between the metals for galvanic corrosion to occur. As shown in the Galvanic Series, tantalum and other exotic alloys have similar noble potentials, so the likelihood of forming a galvanic cell is low.
Second, corrosion depends on the ratio of surface area between the anode and cathode. In cases when the surface area of the anode is larger than the cathode, the likelihood for corrosion decreases. As tantalum is more noble, and thus acts as the cathode, the surface area should be minimized. In fluid systems this can be achieved by using the more noble metal, tantalum, for fittings and valves, while a less noble metal or alloy is used on the tubing.
Tantalum parts can therefore be inserted into the system with confidence, while also saving money by using more cost-effective tubing without compromising on safety or efficiency.
The use of tantalum diffused CVD fittings and valves is an innovative solution for the common issues associated with the use of exotic alloys, namely the high cost, long lead times, and difficulties sourcing these specialty parts, and finally allows the attainable use of the most-corrosion resistant commercially available metal — tantalum.
Print this page